Saturday, December 31, 2011
Tuesday, December 27, 2011
Sotos syndrome
Sotos syndrome (cerebral gigantism) is a rare genetic disorder characterized by excessive physical growth during the first 2 to 3 years of life. The disorder may be accompanied by autism[1] mild mental retardation, delayed motor, cognitive, and social development, hypotonia (low muscle tone), and speech impairments. Children with Sotos syndrome tend to be large at birth and are often taller, heavier, and have larger heads (macrocephaly) than is normal for their age. Signs of the disorder, which vary among individuals, include a disportionately large and long head with a slightly protrusive forehead, large hands and feet, hypertelorism (an abnormally increased distance between the eyes), and downslanting eyes. Clumsiness, an awkward gait, and unusual aggressiveness or irritability may also occur. Although most cases of Sotos syndrome occur sporadically, familial cases have also been reported. It is similar to Weaver syndrome.
http://en.wikipedia.org/wiki/Sotos_syndrome
http://en.wikipedia.org/wiki/Sotos_syndrome
Prader–Willi syndrome
Prader–Willi syndrome (abbreviated PWS) is a rare genetic disorder in which seven genes (or some subset thereof) on chromosome 15 (q 11–13) are deleted or unexpressed (chromosome 15q partial deletion) on the paternal chromosome. It was first described in1956 by Andrea Prader (1919–2001), Heinrich Willi (1900–1971), Alexis Labhart (1916), Andrew Ziegler, and Guido Fanconi of Switzerland.[2] The incidence of PWS is between 1 in 25,000 and 1 in 10,000 live births. The paternal origin of the genetic material that is affected in the syndrome is important because the particular region of chromosome 15 involved is subject to parent of origin imprinting, meaning that for a number of genes in this region only one copy of the gene is expressed while the other is silenced through imprinting. For the genes affected in PWS, it is the paternal copy that is usually expressed, while the maternal copy is silenced. This means that while most people have a single working copy of these genes, people with PWS have no working copy. PWS has the sister syndrome Angelman syndrome in which maternally derived genetic material is affected in the same genetic region.
http://en.wikipedia.org/wiki/Prader%E2%80%93Willi_syndrome
http://en.wikipedia.org/wiki/Prader%E2%80%93Willi_syndrome
Noonan syndrome
Noonan syndrome is a disease passed down through families (inherited) that causes abnormal development in many parts of the body. It used to be called Turner-like syndrome.
Causes, incidence, and risk factors
Noonan syndrome is linked to defects in several genes. Problems with the genes cause certain proteins involved in growth and development to become overactive.
Noonan syndrome is an autosomal dominant condition. This means only one parent has to pass down the faulty gene for the baby to have the syndrome. However, some cases may not be inherited.
What is Russell-Silver syndrome?
Russell-Silver syndrome is a growth disorder characterized by slow growth before and after birth. Babies with this condition have a low birth weight and often fail to grow and gain weight at the expected rate (failure to thrive). Head growth is normal, however, so the head may appear unusually large compared to the rest of the body. Affected children are thin and have poor appetites, and some develop low blood sugar (hypoglycemia) as a result of feeding difficulties. Adults with Russell-Silver syndrome are short; the average height for affected males is about 151 centimeters (4 feet, 11 inches) and the average height for affected females is about 140 centimeters (4 feet, 7 inches).
Many children with Russell-Silver syndrome have a small, triangular face with distinctive facial features including a prominent forehead, a narrow chin, a small jaw, and down-turned corners of the mouth. Other features of this disorder can include an unusual curving of the fifth finger (clinodactyly), asymmetric or uneven growth of some parts of the body, and digestive system abnormalities. Russell-Silver syndrome is also associated with an increased risk of delayed development and learning disabilities.
Failure to thrive - Paediatrics
Definition
FTT is best defined as inadequate physical growth diagnosed by observation of growth over time using a standard growth chart. The National Center for Health Statistics (NCHS) recently released improved growth charts that can be found at www.cdc.gov. While definitions of FTT have varied, most practitioners diagnose FTT when a child's weight for age falls below the fifth percentile of the standard NCHS growth chart or if it crosses two major percentile lines.3 Recent research has validated that the weight-for-age approach is the simplest and most reasonable marker for FTT.4 Other growth parameters that can assist in making the diagnosis of FTT are weight for height and height for age. FTT is diagnosed if a child falls below the 10th percentile for either of these measurements.
Sunday, December 25, 2011
Iron Deficiency Anemia
Definition
Anemia can be caused by iron deficiency, folate deficiency, vitamin B12 deficiency, and other causes. Iron deficiency anemia is due to a shortage of iron. It is characterized by the production of red blood cells that are smaller than normal (microcytic) and appear pale or light colored (hypochromic) when viewed under a microscope. For this reason, the anemia that occurs with iron deficiency is also called hypochromic microcytic anemia.
Description
Iron deficiency anemia is the most common type of anemia throughout the world. In the United States, iron deficiency anemia occurs to a lesser extent than in developing countries because of the higher consumption of red meat and the practice of food fortification (addition of iron to foods by manufacturers). In the United States, iron deficiency anemia is caused by a variety of factors, including excessive losses of iron in menstrual fluids and excessive bleeding into the gastrointestinal tract. In developing countries located in tropical climates, the most common cause of iron deficiency anemia is infestation with hookworm.
Causes and symptoms
Infancy is a period of increased risk for iron deficiency. A human infant is born with a built-in supply of iron, which can be tapped during periods of drinking low-iron milk or formula. Both human milk and cow milk contain rather low levels of iron (0.5-1.0 mg iron/liter). However, about 50% of the iron in human milk is absorbed by an infant, while only 10% of the iron in cow milk is absorbed. During the first six months of life, growth of an infant is made possible by milk in the diet and by the infant's built-in supply. Premature infants have a lower supply of iron. For this reason, it is recommended that pre-term infants (beginning at two months of age) be given oral supplements of 7 mg iron/day, in the form of ferrous sulfate. Iron deficiency can develop when infants are fed formulas that are based on cow milk that has not been fortified. For example, unfortified cow milk is given free of charge to mothers in Chile. This practice prevents general malnutrition, but results in the development of mild iron deficiency.
The normal rate of blood loss in the feces is 0.5-1.0 ml per day. About 60% of persons with cancer of the colon and rectum experience further blood loss in the range of 10 ml/day, which can lead to iron deficiency anemia. The fecal occult blood test is widely used to screen for the presence of cancer of the colon or rectum. In the absence of testing,colorectal cancer may be first detected because of the resulting iron deficiency anemia.
Infection with hookworm can also cause iron deficiency anemia. The hookworm is a parasite that thrives in warm climates, including in the southern United States. A hookworm enters the body through the skin, very commonly through bare feet. The hookworm then migrates to the small intestines where it attaches itself to the villi (small, finger-like structures found on the walls of the intestines, which are used for the absorption of nutrients). Hookworms damage the villi, resulting in blood loss. Further, they produce anticoagulants which promote continued bleeding. Each hookworm can cause the loss of up to 0.25 ml of blood per day.
Bleeding and blood loss through the gastrointestinal tract can also be caused by hemorrhoids, anal fissures, irritable bowel syndrome, aspirin-induced bleeding, blood clotting disorders, and diverticulosis (a condition caused by an abnormal opening from the intestine). Several genetic diseases are characterized by bleeding disorders. These includehemophilia A, hemophilia B, and von Willebrand's disease. Of these, only von Willebrand's disease leads to gastrointestinal bleeding.
The symptoms of iron deficiency anemia include weakness and fatigue. These symptoms result from the lack of function of red blood cells, and the reduced ability of red blood cells to carry iron to exercising muscles. Iron deficiency can also affect other tissues, including the tongue and fingernails. Prolonged iron deficiency can result in changes of the tongue, which may become smooth, shiny, and reddened, a condition known as glossitis. Fingernails may grow abnormally and acquire a spoon-shaped appearance.
Decreased iron intake is a contributing factor in iron deficiency and the resulting iron deficiency anemia. The iron content of some common foods is:
- whole wheat bread (43 mg/kg)
- spinach (33 mg/kg)
- beef (28 mg/kg)
- raisins (20 mg/kg)
- eggs (20 mg/kg)
- lima beans (15 mg/kg)
- potatoes (14 mg/kg)
- canned tuna (13 mg/kg)
- chicken (11 mg/kg)
- peanut butter (6.0 mg/kg)
- tomatoes (3.0 mg/kg)
- cabbage (1.6 mg/kg)
- apples (1.5 mg/kg)
- corn oil (0.6 mg/kg)
It is readily apparent that apples, tomatoes, and corn oil are relatively low in iron, while whole wheat bread, spinach, and beef are relatively high in iron. The assessment of whether a food is low or high in iron can also be made by comparing the amount of that food eaten per day with the recommended dietary allowance (RDA) for iron. The RDA for iron for an adult male is 10 mg/day, while that for an adult woman is 15 mg/day. The RDA during pregnancy is 30 mg/day. The RDA for infants of 0-0.5 years of age is 6 mg/day, while that for infants of 0.5-1.0 year of age is 10 mg/day. RDA values are based on the assumption that a person eats a mixture of plant and animal foods.
The above list of iron values alone may be deceptive, because bioavailability varies. Bioavailability means the percent of iron in the food that is absorbed via the gastrointestinal tract to the bloodstream. Non-absorbed iron is lost in the feces. The bioavailability of iron in fruits, vegetables, and grains is very low, but is much higher in meats. The bioavailability of iron in plants ranges from only 1-10%, while that in meat, fish, chicken, and liver is 20-30%. The most readily absorbable source of iron is human milk, which has a 50% bioavailability.
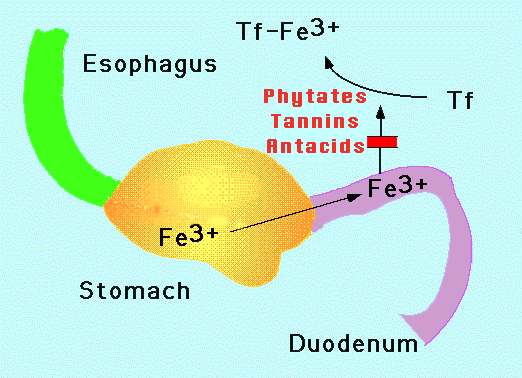
Interactions between various foods also influence the absorption of dietary iron. Vitamin C, for example, increases the absorption of dietary iron. Thus, if rice is consumed with a vitamin C-rich food such as orange juice, then the absorption of the rice's iron is enhanced. The increased use of formulas fortified with both iron and vitamin C has led to a marked reduction in anemia in infants and young children in the United States. In contrast, if rice is consumed with tea, certain chemicals (tannins) in the tea reduce the absorption of iron. Another potent inhibitor of iron absorption is phytic acid, a chemical that occurs naturally in legumes, cereals, and nuts.
Adolescence
Adolescence (from Latin: adolescere meaning "to grow up")[1] is a transitional stage of physical and mental human developmentgenerally occurring between puberty and legal adulthood (age of majority),[1][2] but largely characterized as beginning and ending with the teenage stage.[2][3][4] According to Erik Erikson's stages of human development, for example, a young adult is generally a person between the ages of 20 and 40, whereas an adolescent is a person between the ages of 13 and 19.[3][4] Scholars have found it incredibly difficult to agree upon a precise definition of adolescence, because it can be approached from so many angles
http://en.wikipedia.org/wiki/Adolescence
http://en.wikipedia.org/wiki/Adolescence
Importance of iron regulation- Excretion
The human body needs iron for oxygen transport. That oxygen is required for the production and survival of all cells in our bodies. Human bodies tightly regulate iron absorption and recycling. Iron is such an essential element of human life, in fact, that humans have no physiologic regulatory mechanism for excreting iron. Most humans prevent iron overload solely by regulating iron absorption. Those who cannot regulate absorption well enough get disorders of iron overload. In these diseases, the toxicity of iron starts overwhelming the body's ability to bind and store it. [2]
http://en.wikipedia.org/wiki/Human_iron_metabolism#Importance_of_iron_regulation
Despite the fact that iron is the second most abundant metal in the earth's crust, iron deficiency is the world's most common cause of anemia. When it comes to life, iron is more precious than gold. The body hoards the element so effectively that over millions of years of evolution, humans have developed no physiological means of iron excretion. Iron absorption is the sole mechanism by which iron stores are physiologically manipulated.
http://sickle.bwh.harvard.edu/iron_absorption.html
http://en.wikipedia.org/wiki/Human_iron_metabolism#Importance_of_iron_regulation
Despite the fact that iron is the second most abundant metal in the earth's crust, iron deficiency is the world's most common cause of anemia. When it comes to life, iron is more precious than gold. The body hoards the element so effectively that over millions of years of evolution, humans have developed no physiological means of iron excretion. Iron absorption is the sole mechanism by which iron stores are physiologically manipulated.
http://sickle.bwh.harvard.edu/iron_absorption.html
INTERACTIONS BETWEEN SELENIUM AND IODINE
Selenium and iodine are two minerals which are critically important in the proper functioning of the thyroid. While the importance of iodine has been known a long time, the importance of selenium has only been discovered and explored since 1990. Much research is presently being conducted on the functions of these two minerals in thyroid function and it is becoming clear that there is an interaction between the two. Iodine has a seemingly simple role in the thyroid-it is incorporated into the thyroid hormone molecule.
A deficiency of iodine will cause hypothyroidism and if this is severe and occurs during pregnancy, the offspring will be mentally damaged and is called a cretin. Cretinism, or myxeodematous cretinism as it is sometimes called, is not only caused by an iodine deficiency, but is also influenced by a selenium deficiency. Iodine apparently has just one function in the body-in the thyroid.
Selenium, on the other hand, performs many functions. At the beginning of the 1990s it was discovered that the deiodinase enzymes which convert T4 (thyroxin, the thyroid prohormone) into T3 (triiodothyronine, the cellularly active hormone) and also convert T3 into T2, thereby degrading it, are selenium enzymes (formed with the amino acid cysteine). This discovery has led to a lot of research studies on the effects of selenium, iodine, and their interactions.
Selenium also performs other important roles in the body. The most important of these is probably as its role as the body's best antioxidant (anti-peroxidant). It performs this role as part of glutathione peroxidase (GSHPx or GPX). As part of GPX, selenium prevents lipids and fats from being peroxidized (oxidized), which literally means that it prevents fats from going rancid (this can be seen on your skin as "age spots" or "liver spots" (autopsies show that skin "liver spots" are accompanied by similar spots of peroxidized fats in the liver.) Therefore selenium protects all of the cellular membranes, which are made up of fats, from peroxidation. Peroxidation of cellular membranes reduces the ability of the membrane to pass nutrients including minerals and vitamins, so selenium deficiency is the first step toward developing the many problems caused by nutrient deficiencies.
Joel Wallach considers a selenium deficiency combined with high intake of vegetable oils (salad dressings, margarine, cooking oils) as the "quickest route to a heart attack and cancer." It seems that the body uses a lot of selenium to protect the fats from peroxidation. Polyunsaturated fats which are hydrogenated or heated become the same as rancid fats and large amounts of selenium are then needed to protect the body. Consumption of these dietary fats can thus lead to a selenium deficiency.
Selenium is also essential for the production of estrogen sulfotranserfase which is the enzyme which breaks down estrogen. A deficiency of selenium can thus lead to excessive amounts of estrogen, which may depress thyroid function, and also upset the progesterone-estrogen balance.
Wallach also lists other effects of selenium deficiency: anemia (red blood cell fragility), fatigue, muscular weakness, myalgia (muscle pain), muscular dystrophy (white muscle disease in animals), cardiomyopathy (sudden death in athletes), heart palpitations, irregular heartbeat, liver cirrhosis, pancreatitis, Lou Gehrig's and Parkinson's diseases (mercury toxicity), Alzheimer's Disease (high intake of vegetable oil), sudden infant death syndrome (and possibly "breathlessness" in adults, jj), cancer, multiple sclerosis, and sickle cell anemia.
Selenium is essential for the production of testosterone. A deficiency seems to be involved in osteoarthritis. I've found studies linking selenium deficiency to alopecia (hair loss) and to degeneration of the knee joint (seen in Kashin-Beck disease). Since selenium is necessary to produce GPX which is a major detoxifier of man-made and environmental toxins, selenium deficiency can lead to chemical and drug sensitivities.
These are some of the non-thyroidal effects of selenium deficiency. The effects of selenium deficiency on thyroidal health is even more interesting. One study I read indicated that in experimental animals, selenium deficiency will increase T3 in the heart. This may be the reason that selenium deficiency causes heart palpitations and rapid heart beat, which is common in thyroid disease.
While we've seen that selenium deficiency will interfere with T4 to T3 conversion and lead to functional hypothyroidism (low T3 phenomenon), selenium plays another vital role in the thyroid as part of GPX. During the production of thyroid hormone, hydrogen peroxide (H2O2) is produced. H2O2 is important for the production of thyroid hormone, but excessive amounts lead to high production of thyroxin (T4) and also damage to the cells of the thyroid. GPX plays the extremely vital role of degrading H2O2 and thereby limiting hormone production and preventing damage to the thyroid cells. This seems to be the main way in which selenium protects the thyroid from sustaining damage which can lead ultimately to cancer.
Without selenium, the thyroid gland becomes damaged and it is through this mechanism that the main selenium and iodine interactions are found. An iodine deficiency will cause goiter, an enlargement of the thyroid gland produced by the body in an attempt to increase hormone production from limited amount of iodine. Selenium deficiency increases the weight of the thyroid in experimental animals, and a selenium deficiency combined with an iodine deficiency leads to a further increase in thyroidal weight (bigger goiter). In African countries like Zaire, there are areas where both iodine and selenium are very scarce in the soil (these deficiencies seem to run parallel in most areas). Consequently a high percentage of the people have goiters and hypothyroidism. An experimental attempt was made to correct the selenium deficiency and the result was that the hypothyroidism was made WORSE in the hypos and it produced hypothyroidism in some euthroid subjects. This was entirely unexpected and the experimenters issued a warning about supplementing with selenium (and not iodine) when both deficiencies exist concurrently.
The body has a compensatory mechanism to maintain T3 levels when iodine is deficient--it increases the production of the deiodinase Type I enzyme (DI-I). This is not a small increase, but has been shown in cattle to be an increase of 10-12 times. This increase in ID-I increases the conversion of the existing T4 to T3 to maintain T3 levels, but also increases the conversion of T3 to T2 (the degraded by-product of T3). Because of the iodine deficiency, T4 is not replenished and T3 ultimately decreases from the lack of sufficient T4 leading to a worsening of the hypothyroidism.
This result is made worse by another phenomenon which hasn't been thoroughly studied: a selenium deficiency causes an iodine deficiency to get worse. This may be a protective adaptation by the body to limit the damage caused to the thyroid when selenium is deficient and iodine is adequate. Let's examine this part of the interaction.
We've all heard that many doctors tell hypo patients, especially those with Hashimoto's thyroiditis, not to take iodine because it can aggravate their condition. The reason seems to be that selenium protects the thyroid gland from oxidative damage and this damage can increase significantly if iodine is supplemented. Taking iodine will increase thyroid hormone production and the production of H2O2 which damages the thyroidal cells. The lack of selenium prevents GPX from being able to protect the cells from this oxidative damage. While I doubt if most doctors realize why iodine should be restricted (it certainly seemed counter-intuitive to me at first), they have learned through experience that iodine can increase the thyroid damage in Hashimoto's. The information that selenium should be supplemented along with iodine is so new that most of them are unaware of it.
Here's what we have: Studies have shown that if iodine is low, selenium must also be kept low to prevent the hypothyroidism from becoming worse (from increased DI-I and T4 depletion, as explained above.) So if both minerals are low, then the person is hypo and gets a goiter, but the damage to the thyroid is kept to a minimum. More severe problems happen when either selenium or iodine is high and the other is low. If selenium is high and iodine low, then T4 to T3 to T2 conversion is accelerated without T4 being replenished, leading to a worsening of the hypoT. If iodine is high and selenium is low, then H2O2 is not degraded by GPX. Since H2O2 drives the thyroid hormone production, then the thyroid over-produces thyroid hormone (Grave's hyperthyroidism), the thyroid is damaged from the oxidation by the H2O2, and the end result is that the damaged thyroid ultimately decreases activity and hypothyroidism results (Hashimoto's thyroiditis). This could explain the observed progression of Grave's to Hashimoto's.
If a selenium deficiency causes an iodine deficiency, leaving you both selenium and iodine deficient, and supplementing with either selenium or iodine causes severe problems, then the only solution is to supplement both selenium and iodine simultaneously and gradually. Even then you could experience an immediate boost (from increased conversion of T4 to T3) with a subsequent letdown (lack of T4 production because of insufficient iodine or other necessary nutrient). You have to be prepared to ride out the tough times and continue increasing the selenium and iodine until those two deficiencies are corrected and the respective metabolic pathways are back working properly.
Everything that I've read about selenium indicates that it is absolutely essential for proper functioning of the thyroid. A deficiency of selenium may lead to either hyperthyroidism or hypothyroidism. I've always wondered if high intake of selenium can lead to hyperthyroidism and finally found someone who did the experiment. They found that a high intake of selenium will not increase T4 production and lead to hyperthyroidism.
If a person has hyperT, then it looks like taking selenium without iodine will result in a decrease in production of T4 (although there may be an initial transient increase in T4 to T3 conversion and hence higher T3). I would suggest to start with a small amount of selenium methionine (about 50 mcg) and gradually increase it. I cannot see any way that thyroid function can be normalized without selenium.
For hypos the important message is that a selenium deficiency may cause an iodine deficiency, so that even though you are taking iodine you may not be assimilating it unless selenium is also being taken. This would explain how people can have iodine deficiencies even though salt and many foods have iodine added. Supplement with both iodine and selenium. I would recommend starting with 100 mcg of selenium and one kelp tablet and gradually work up to 400-600 mcg of selenium and 2-4 tablets of kelp. [Note from the Green Willow Tree: Our research indicates that there is an upper safety limit of 400 mcg./day for selenium, and we do not recommend taking more than that amount. Also, kelp is extremely high in iodine, which is good for the short term. However, excess iodine consumption long term can actually depress thyroid function. Dulse, bladderwrack, and Irish moss--the seaweeds found in Thyodine--are safer, in our opinion, for long term use.]
http://www.greenwillowtree.com/Page.bok?file=selenium.iodine.html
Iron Deficiency Anemia Clinical Presentation
Children deficient in iron may exhibit behavioral disturbances. Neurologic development is impaired in infants and scholastic performance is reduced in children of school age. The intelligence quotients (IQs) of schoolchildren deficient in iron are reported to be significantly lower than those of their nonanemic peers. Behavioral disturbances may manifest as an attention deficit disorder. Growth is impaired in infants with iron deficiency. The neurologic damage to an iron-deficient fetus results in permanent neurologic injury and typically does not resolve on its own. Iron repletion stabilizes the patient so that his or her status does not further decline.
http://emedicine.medscape.com/article/202333-clinical#aw2aab6b3b3
http://emedicine.medscape.com/article/202333-clinical#aw2aab6b3b3
Premature Infants
Preterm infants are particularly susceptible to iron deficiency anemia due to the lack of iron stores built up in the 3rd trimester and also due to the large number of blood draws required during their hospitalizations.
Intrauterine Growth Restriction (IUGR) and Iron deficiency anaemia
http://hospital.blood.co.uk/library/pdf/Anaemia_and_Womens_Health.pdf
What are the effects / risks
• Increased risk of IUGR and
premature birth (with anaemia
present at beginning of
pregnancy)
• Possible problems associated
with being cared for by a
weak / tired mother
http://www.chp.edu/CHP/P02462
What are the effects / risks
• Increased risk of IUGR and
premature birth (with anaemia
present at beginning of
pregnancy)
• Possible problems associated
with being cared for by a
weak / tired mother
http://www.chp.edu/CHP/P02462
What causes intrauterine growth restriction (IUGR)?
Intrauterine growth restriction results when a problem or abnormality prevents cells and tissues from growing or causes cells to decrease in size. This may occur when the fetus does not receive the necessary nutrients and oxygen needed for growth and development of organs and tissues, or because of infection. Although some babies are small because of genetics (their parents are small), most IUGR is due to other causes. Some factors that may contribute to IUGR include the following:
- Maternal factors:
- high blood pressure
- chronic kidney disease
- advanced diabetes
- heart or respiratory disease
- malnutrition, anemia
- infection
- substance abuse (alcohol, drugs)
- cigarette smoking
- Factors involving the uterus and placenta:
- decreased blood flow in the uterus and placenta
- placental abruption (placenta detaches from the uterus)
- placenta previa (placenta attaches low in the uterus)
- infection in the tissues around the fetus
- Factors related to the developing baby (fetus):
- multiple gestation (twins, triplets, etc.)
- infection
- birth defects
- chromosomal abnormality
Etiology
Many different factors cause IUGR, but they may be divided into two large categories, based on etiology. These categories include fetoplacental factors and maternal factors. Within the categories of maternal and fetoplacental factors are many specific causes (Table 1).
TABLE 1
Conditions Associated with Intrauterine Growth Retardation
Conditions Associated with Intrauterine Growth Retardation
Medical |
Chronic hypertension |
Preeclampsia early in gestation |
Diabetes mellitus |
Systemic lupus erythematosus |
Chronic renal disease |
Inflammatory bowel disease |
Severe hypoxic lung disease |
Maternal |
Smoking |
Alcohol use |
Cocaine use |
Warfarin (Coumadin, Panwarfin) |
Phenytoin (Dilantin) |
Malnutrition |
Prior history of pregnancy with intratuterine growth retardation |
Residing at altitude above 5,000 feet |
Infectious |
Syphilis |
Cytomegalovirus |
Toxoplasmosis |
Rubella |
Hepatitis B |
HSV-1 or HSV-2 |
HIV-1 |
Congenital |
Trisomy 21 |
Trisomy 18 |
Trisomy 13 |
Turner's syndrome |
Historically, IUGR has been categorized as symmetric or asymmetric. Symmetric IUGR refers to fetuses with equally poor growth velocity of the head, the abdomen and the long bones. Asymmetric IUGR refers to infants whose head and long bones are spared compared with their abdomen and viscera. It is now believed that most IUGR is a continuum from asymmetry (early stages) to symmetry (late stages).
Maternal causes of IUGR account for most uteroplacental cases. Chronic hypertension is the most common cause of IUGR. Moreover, the infants of hypertensive mothers have a three-fold increase in perinatal mortality compared with infants with IUGR who are born of normotensive mothers. Because of their significant risk, one author6 recommends delivering these infants by 37 weeks of gestational age.
Preeclampsia causes placental damage that results in uteroplacental insufficiency. The pathogenic mechanism is thought to be a failure of trophoblastic invasion by maternal spiral arterioles by 20 to 22 weeks of gestation.1 This failure causes luminal narrowing and medial degeneration, leading to diminished blood flow to the developing infant. Consequently, these infants fail to grow normally.
Infectious causes of fetal growth delay account for about 10 percent of all cases of IUGR. These causes include the “TORCH” group: Toxoplasma gondii, rubella, cytomegalovirus and herpes simplex virus types 1 and 2. Other potential pathogens include hepatitis A and hepatitis B, parvovirus B19, human immunodeficiency virus (HIV) and Treponema pallidum (syphilis).
Maternal prepregnancy weight and weight gain during pregnancy are considered strong indicators of birth weight.7 During World War II, a population of women in Leningrad who underwent prolonged malnutrition delivered infants with an average birth weight of 400 to 600 g (14 to 21 oz) less than expected.5 In a later study of Guatemalan Indians,8 it was found that protein malnutrition occurring before 26 weeks of gestation resulted in IUGR. The current consensus is that a maternal weight gain of less than 10 kg (22 lb) by 40 weeks of gestation is clearly a risk factor for IUGR.3
Maternal smoking may be the cause of 30 to 40 percent of U.S. cases of IUGR. One study9 found a dose-dependent decrease in fetal weight with an increasing number of cigarettes smoked each day (a 7.4 g [0.26 oz] decrease for each cigarette smoked per day). Another study10 found that women who smoked 11 or more cigarettes daily had infants weighing 330 g (11.5 oz) less than predicted and measuring 1.2 cm shorter than control subjects.
Early use of alcohol by the pregnant mother may lead to fetal alcohol syndrome, while second- or third-trimester use may result in IUGR. As little as one to two drinks per day have been shown to result in a growth-delayed child.11 Not surprisingly, maternal cocaine use has been linked to IUGR, as well as to reduced head circumference. Other drugs associated with IUGR include steroids, warfarin (Coumadin, Panwarfin) and phenytoin (Dilantin).
Intrauterine growth retardation occurs 10 times more frequently in twin deliveries than in single gestations. The incidence of IUGR in twins is about 15 to 25 percent.5 Decreased birth weight is second only to respiratory distress syndrome as a cause of infant mortality in twins. Reasons for IUGR in twin pregnancies include poor placental implantation, placental crowding and twin-to-twin transfusion.
Subscribe to:
Posts (Atom)

,_de_Juan_Carre%C3%B1o_de_Miranda..jpg)